
A matchstick looks simple, almost insignificant. Yet behind its small size lies a carefully engineered system that combines chemistry, physics, and design efficiency. For more than a century, the matchstick has remained one of the most reliable fire-starting tools ever created, with very little change in its structure or cost.
This article dives into the inner workings of a matchstick, the chemistry that sparks its flame, and the fascinating history behind its invention – all presented in a clear, educational style for curious minds.
Fire and the Three Fundamental Requirements

Fire does not occur randomly. From a scientific standpoint, combustion requires three essential components, commonly known as the fire triangle:
Heat – energy to start the reaction
Fuel – a substance that can burn
Oxygen – to sustain the reaction
A matchstick is designed to bring these three components together only at the moment of use. Until then, each element remains safely separated. This controlled interaction is the foundation of the matchstick’s effectiveness and safety.
The Hidden Engineering Inside a Matchstick
Although it appears basic, a modern safety matchstick consists of multiple chemically distinct zones, each with a specific function.
1. The Matchstick Head (Reactive Zone)
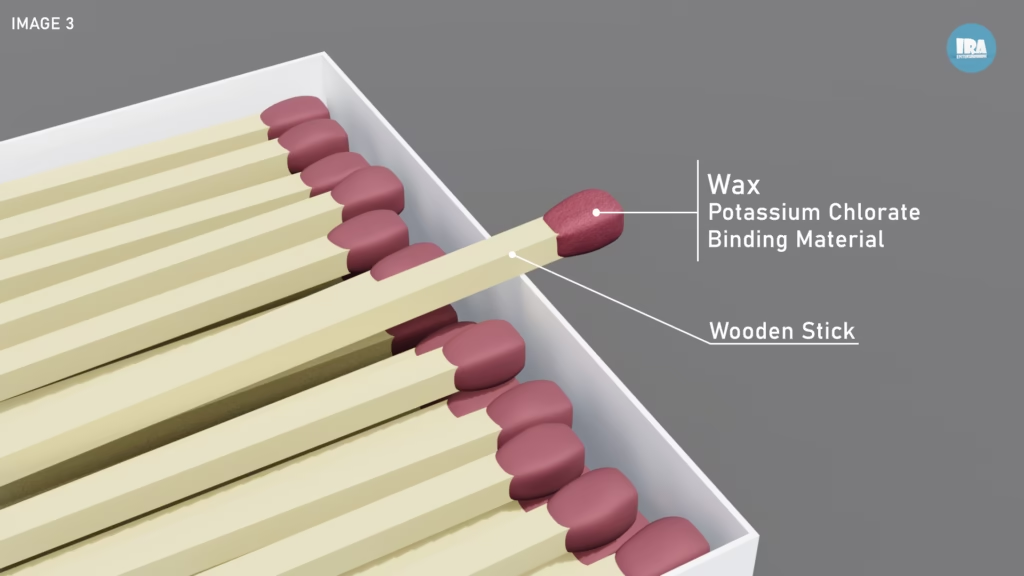
The tip of the matchstick contains a mixture of substances that act as:
Fuel sources (such as sulfur and wax)
Oxidizing agents (commonly potassium chlorate)
Binding materials to hold the structure together
These materials do not ignite on their own under normal conditions. They are designed to respond only when sufficient heat is applied.
2. The Matchbox Striking Surface

The striking strip on the side of a matchbox is coated with red phosphorus, an intentionally stable form of phosphorus.
Crucially, this material is not placed on the matchstick itself. This separation is what makes modern matches “safety matches,” reducing accidental ignition during storage or transport.
3. The Wooden Stick (Sustained Fuel)
Once ignition begins, the wooden stick (See image 3) becomes the primary fuel source. Treated wood ensures:
Controlled burning
Predictable flame size
Sufficient burn time for practical use
What Happens When a Match Is Struck?
From a physics and chemistry perspective, striking a match initiates a chain reaction rather than a single event.
Step 1: Friction Creates Heat

When the match is rubbed against the striking surface, friction generates localized heat at the contact point.
Step 2: Phosphorus Transformation
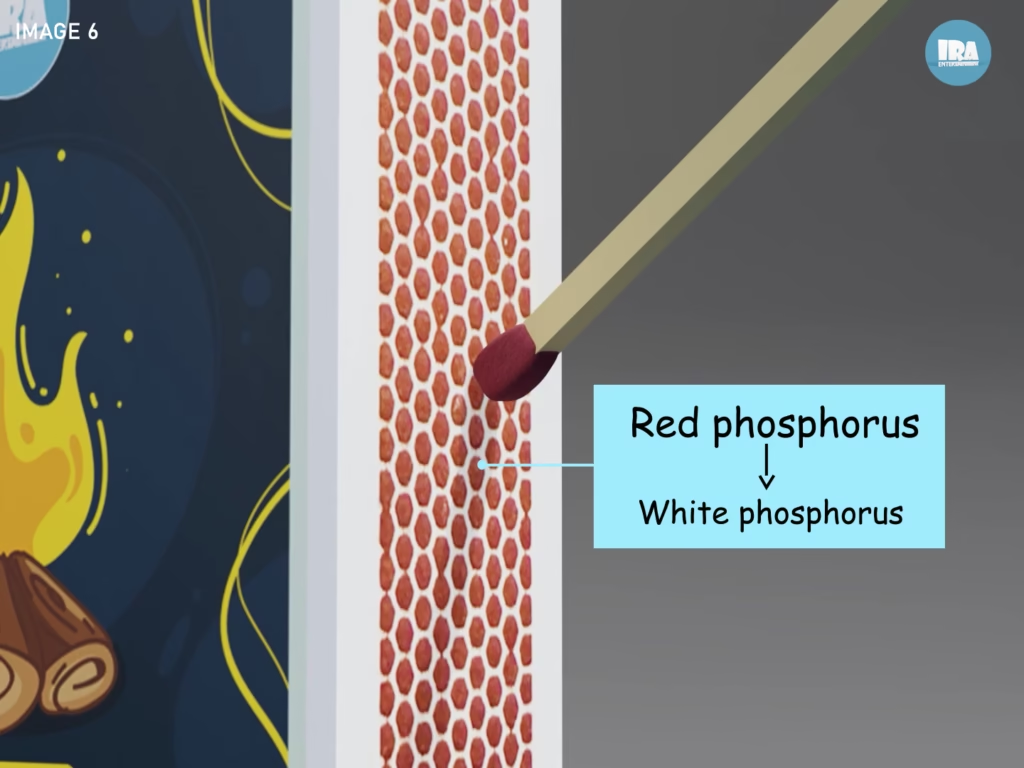
The heat causes a small amount of red phosphorus on the striking surface to transform into white phosphorus, a much more reactive form. This transformation produces a brief spark.
Step 3: Oxygen Release and Combustion
Simultaneously, heat activates the oxidizing agent in the match head. This releases oxygen, allowing the fuel components to ignite rapidly.
Step 4: Flame Transfer to the Stick

Once the head ignites, the flame spreads to the wooden stick, which then sustains the fire independently.
This entire sequence occurs in a fraction of a second and demonstrates precise control over chemical energy release.
Why Matchsticks Are Considered a Design Success
From an engineering standpoint, the matchstick is remarkable for several reasons:
Minimal materials
Low manufacturing cost
High reliability
Long shelf life
No external power source required
Few inventions combine such simplicity with consistent global usefulness.
Fire Before the Matchstick
Long before the invention of matchsticks, humans used various methods to create fire, each requiring patience, skill, and careful preparation.
Flint and Steel: One of the earliest controlled fire-making methods involved striking a piece of flint against steel to create sparks. These sparks would then ignite a small pile of tinder, such as dry grass or shredded wood. While effective, this method required practice, precision, and a steady hand.
Burning Embers: Another common technique involved maintaining a fire continuously and transporting burning embers from one location to another. This method was labor-intensive, and if the embers went out, it could take hours to reignite a flame.
Friction-Based Fire Tools: In many cultures, friction methods like the bow drill or hand drill were used to generate heat through rapid rubbing of wood pieces. This technique relied on creating enough friction to ignite small particles, which could then start a fire. It demanded considerable strength, patience, and knowledge of the right materials.
These traditional fire-starting techniques highlight just how valuable and transformative the invention of the matchstick became. With matches, creating fire no longer required specialized skill or constant attention — a small, portable tool could reliably produce a flame anytime, anywhere.
Who Invented the Matchstick?

The invention of the modern matchstick is credited to John Walker, an English chemist, in 1826. Walker discovered the principle of friction ignition accidentally while experimenting with chemical compounds in his laboratory. When a chemical-coated wooden stick was scraped against a rough surface, it ignited—revealing a new, simple way to produce fire.
Walker’s invention, known as “friction lights,” was the first practical match that could be ignited by rubbing. Although effective, these early matches were crude, produced sparks, and emitted unpleasant odors. Notably, Walker never patented his invention, allowing others to rapidly improve upon the idea.
In the decades that followed, chemists refined the matchstick by improving ignition reliability and reducing danger. Early versions using white phosphorus were highly flammable and toxic, leading to serious health risks. This eventually prompted the development of the safety match in the mid-19th century, where phosphorus was moved to the striking surface instead of the match head—a design that remains the global standard today.
The matchstick, as a result, is not the product of a single moment of invention, but a progression of scientific refinement—beginning with Walker’s discovery and perfected through thoughtful chemical engineering.
Matches and the Age of Smoking
Interestingly, matchsticks became widespread after cigarettes gained popularity, not before. The growing demand for a quick, portable fire source accelerated matchstick development during the 19th century.
The first chemical matches were effective but dangerous, often igniting accidentally and containing toxic substances.
The Safety Match Revolution
The introduction of the safety match—where phosphorus was separated from the match head—marked a major breakthrough. This design significantly reduced risks and became the global standard.
Despite technological advancements in many areas, the safety match has remained largely unchanged ever since.
Why the Matchstick Design Has Barely Changed
Unlike many inventions that evolve rapidly, the matchstick reached functional perfection early.
Reasons include:
No complex mechanics
No electronic dependency
Universal usability
Compatibility with basic materials
From an economic perspective, the matchstick delivers maximum utility for minimal cost, making redesign unnecessary.
Matchsticks as an Educational Tool
In science education, matchsticks are often used to demonstrate:
Energy transformation
Oxidation and reduction reactions
Heat transfer
Controlled combustion
In 3D educational content, matchsticks offer an excellent subject for visualizing microscopic chemical processes in an accessible way.
Environmental and Practical Considerations
While matchsticks are disposable, they are typically made from:
Biodegradable wood
Small quantities of chemicals
Minimal packaging
Compared to many modern ignition tools, their environmental footprint remains relatively low.
Frequently Asked Questions
1) Why doesn’t a matchstick ignite without striking?
Because heat is required to initiate the chemical reactions, and that heat is only generated through friction.
2)Why is phosphorus not placed on the match head?
Separating phosphorus improves safety and prevents accidental ignition.
3)Why hasn’t the matchstick been replaced completely?
Despite alternatives like lighters, matchsticks remain reliable, inexpensive, and independent of fuel refills or mechanisms.
4)Is a matchstick an example of controlled combustion?
Yes. It is a textbook example of controlled, localized combustion.
Final Thoughts
The matchstick proves that great inventions don’t need complexity. By combining fundamental scientific principles with thoughtful design, it has remained relevant for generations.
In a world of rapidly changing technology, the matchstick stands as a reminder that efficiency, simplicity, and purpose-driven engineering can outlast trends.
Sometimes, the smallest tools carry the smartest ideas.
About the Author
This article was prepared by the IRA Studios editorial team, creators of high-quality 3D educational visualizations designed to simplify complex ideas through clear and engaging visuals.
Watch the Full 3D Animation
To explore all of these processes in 3D, check out our detailed 3D Animation video.
Watch it in Malayalam!
Want to learn more through visual storytelling? Check out our detailed 3D explanation blog on What Is a Treadwheel Crane?
Useful Links :